About MelaGenix

About MELAGENIX
MELAGENIX is a quantitative RT-PCR assay based on expression of eleven genes (eight prognostic and three reference genes) in and around primary melanoma tissue.
Patients are allocated to two groups:
- A low score means the cancer has a lower chance of returning
- A high score means the cancer has a higher chance of returning
MelaGenix validation data in stage II melanoma (Amaral, Eur J Canc (2020))

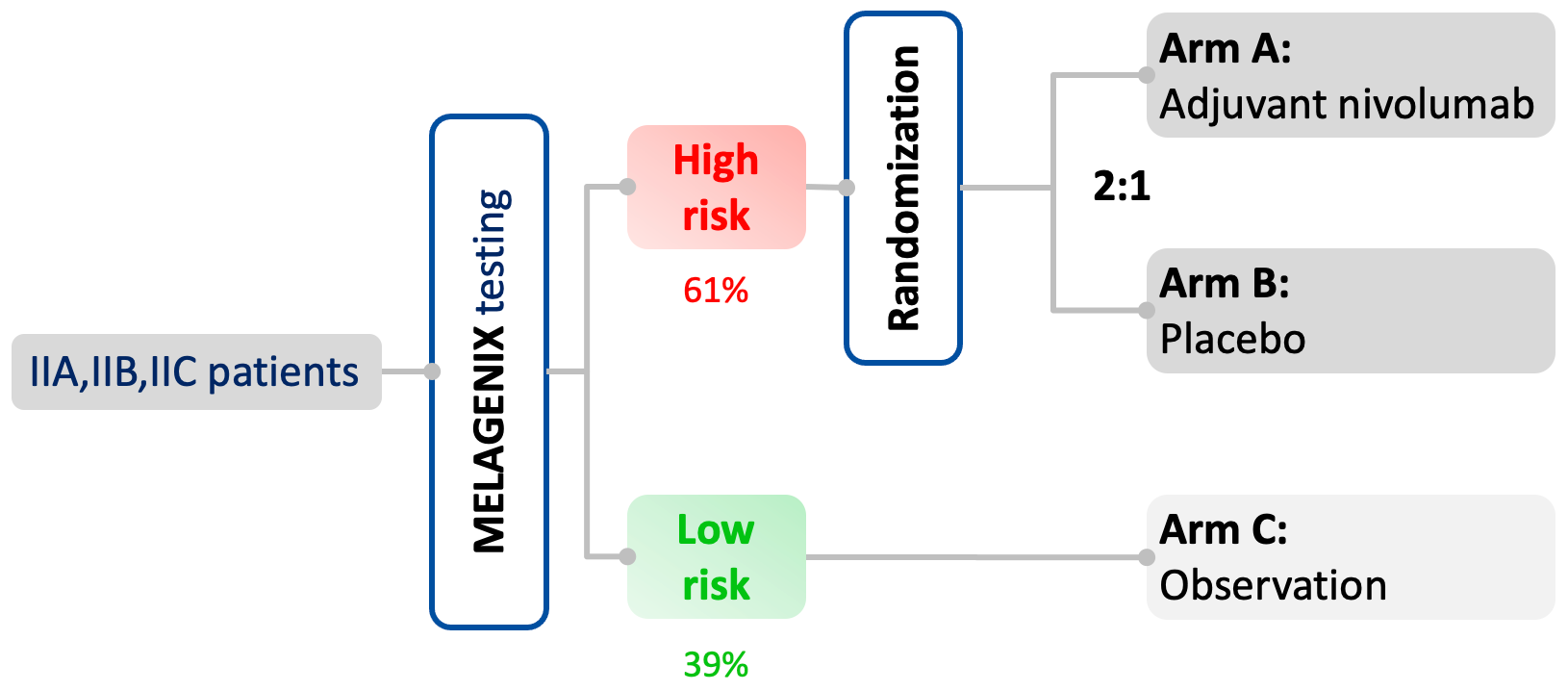
The NivoMela trial
Lead by Prof Dr Dirk Schadendorf, the phase III NivoMela (Nivolumab + MelaGenix) trial is the first clinical trial in melanoma to select patients for adjuvant treatment based on individualized risk for relapse.
Initiated in 2020, it has fully enrolled around 400 stage II patients and randomized the "MelaGenix High Risk" group to adjuvant treatment with nivolumab vs. placebo (2:1).
Clinical evidence:
AJCC Stage II patients
Download publication from European Journal of Cancer
Amaral et al. (2020) clinically validated MELAGENIX
in a large collective of AJCC stage II patients.
Results: MELAGENIX was clinically validated as an independent prognostic factor for RFS&MSS in patients with AJCC stage II cutaneous melanoma. Specifically, the results showed that at 92%, the 10-year MSS in the MELAGENIX low risk group is equivalent to the 5-year MSS of a IIA patient.
Survival by AJCC stages and MELAGENIX
risk group
| 5-y MSS | 10-y MSS | % of cohort | |
|---|---|---|---|
| All | 86% | 75% | 100% |
| IIA | 92% | 84% | 48% |
| IIB | 85% | 69% | 32% |
| IIC | 70% | 52% | 20% |
| low score | 92% | 92% | 37% |
| high score | 82% | 67% | 63% |
Contact:
Phone: +49 (69) 2731595-00
info@neracare.com
NeraCare GmbH
Eschenheimer Anlage 26
D-60318 Frankfurt
Managing directors:
Daniel von Janowski, Dr. Matthias Ackermann
Imprint
Copyright 2020 © All Rights reserved.